Easy read Differential Scanning Calorimetry (DSC) in 2021
know a short simple description about the instrument Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry is a system wherein the heat flux to the example is checked against time or temperature while the temperature of the sample, in a predetermined air, is customised. Practically speaking, the distinction in heat flux to a pan containing the example and an empty pan is checked. The instrument utilised is a differential scanning calorimeter or DSC. The DSC is economically accessible as a power-compensating DSC or as a heat-flux DSC.
Principle
When a sample experiences a physical change, for example, a stage transition, pretty much heat should stream to it than to the reference (commonly an empty sample pan) to keep up both at a similar temperature. Regardless of whether a higher amount of less heat must stream to the sample relies upon whether the procedure is exothermic or endothermic. For e.g.as an active sample melts to the fluid, it will require more heat streaming to the sample to expand its temperature. At a similar rate as the reference.
This is because of the retention of heat by the sample as it experiences the endothermic stage transition from strong to fluid. In like manner, as the sample undergoes exothermic processes. (For example, crystallisation) Less heat is required to raise the sample temp. By watching the distinction in heat stream between the sample and reference, DSC can quantify the measure of heat retained or discharge during such transition.
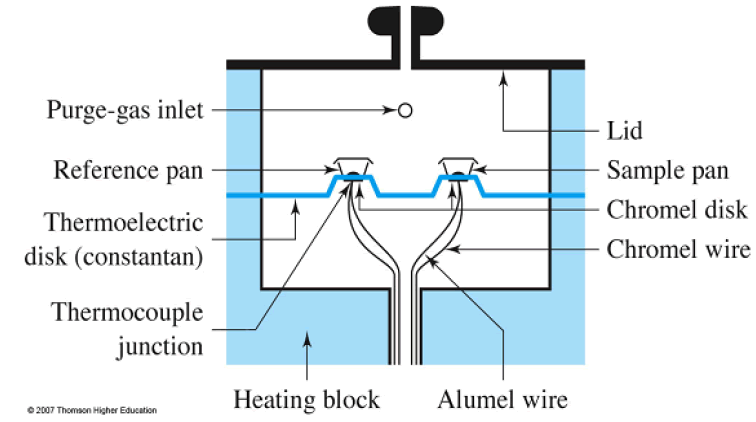
Heat Flux DSC
Heat Flux DSC contains the sample and reference holder, the heating resistor, the heat sink, and the heater. The heat of the heater is provided into the sample and the reference through the heat sink and heat resistor. Heat stream is corresponding to the heat distinction of heat sink and holders. The heat sink has enough heat limit contrasted with the sample. If the sample happens endothermic or exothermic marvels, for example, transition and response, these endothermic or exothermic wonders are repaid by a heat sink.
Therefore, the temperature distinction between the sample and the reference is kept steady. The distinction between the measure of heat provided to the sample and the reference is relative to the temperature contrast of the two holders. By adjusting the standard material, the obscure sample quantitative estimation is reachable.
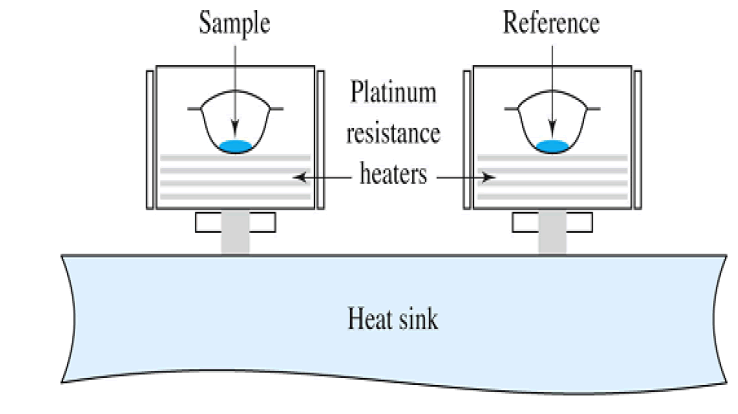
Power Compensation DSC
A system where the distinction of thermal energy that is applied to the sample and the reference material per unit of time is estimated as a component of the temperature to even out their temperature, while the temperature of the sample unit, framed by the sample and reference material, is shifted in a predetermined program. The power-compensating DSC has two about indistinguishable (as far as heat misfortunes) estimating cells, one for the sample and one reference holder.
The two cells are heated with independent heaters, and their temperatures are estimated with isolated sensors. The temperature of the two cells can straightly differ as an element of time being constrained by a usual temperature control circle. A second-differential-control circle modifies the power contribution when a temperature distinction begins to happen because of some exothermic or endothermic procedure in the sample. The differential power signal is recorded as an element of the actual sample temperature.
Typical DSC Curve
The result of a DSC analysis is a curve of heat flux versus temperature or versus time. This curve can be utilised to compute enthalpies of transitions, which is finished by coordinating the pinnacle comparing to a given transition. The enthalpy of transition can be communicated utilising equation:
ΔH = KA
Where, ΔH = enthalpy of transition,
K= calorimetric constant,
A= area under the peak.
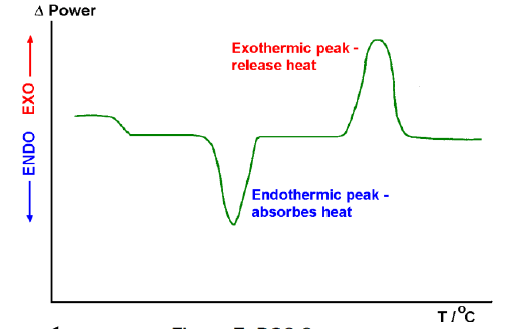
Calorimetric consistent changes from instrument to instrument and can be controlled by breaking down a well-described material of known enthalpies of transition. The zone under the peak is legitimately corresponding to heat consumed or developed by the reaction. The height of the peak is directly proportional to the rate of the reaction.
The Factors Affecting DSC Curve are as follows
Instrumental factors
• Furnace heating rate
• Recording or chart speed
• Furnace atmosphere
• The geometry of the sample holder/location of sensors
• The sensitivity of the recording system
• Composition of sample containers
Sample characteristics
• Amount of sample
• Nature of sample
• Sample packing
• The solubility of evolved gases in the sample
• Particle size
• Heat of reaction
• Thermal conductivity
Applications
- Determination of Heat Capacity
- The Glass Transition Temperature
- Crystallisation
- Melting
- Drug analysis
- Polymers
- Food science
Now that you have read about Differential Scanning Calorimetry. Check out our course on Ampersand Academy Read this interesting article about Nanotechnology & Nanomedicine.