Know in detail about the instrument thermogravimetric analysis
Prepared by Dr. J . Helan Chandra
Thermogravimetric analysis (TGA) is one of the thermal methods in which the change in weight of the sample kept under vacuum is measured over time with respect to change in temperature.
Principle
It is performed by escalating the temperature of a sample kept inside the furnace gradually and the mass is measured with the balance which remains outside of the furnace.
Mechanism of change in mass:
In TGA, weight loss is observed may be due to decomposition (Breaking Chemical bonds), evaporation (Volatilization/ vaporization/ Sublimation) reduction and desorption. A gain in weight may be due to oxidation and adsorption/absorption.
Application
- Characterization study
- To measure thermal stability
- To identify the purity of the sample
- To determine the moisture content of the sample
- To determine the transition temperature
- Examination study
- To study kinetics for the sample disintegration
- To analyse corrosion of the sample with respect to oxidation
- Analysis of Thermo-stable polymers
Types of TGA
It is classified into three types.
- Isothermal or static thermogravimetry- In this technique, at a constant temperature, Change in weight of the sample is measured with respect to change in time.
- Quasistatic thermogravimetry- In this technique, the sample is heated to a constant weight each of series of increasing temperatures.
- Dynamic thermogravimetry- In this technique, the sample is heated in an environment whose temperature is changed in a linear manner.
Factors affecting the TG curve
Instrumental factors Sample Characteristics
Furnace heating rate Weight of the sample
Furnace atmosphere Sample particle size
Instrumentation
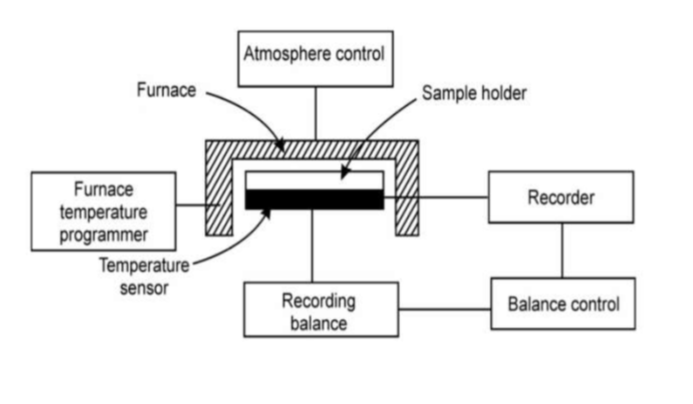
- Furnace: Temperature of the sample in the holder kept in the furnace is raised slowly. Change in temperature of the sample and the corresponding weight is taken in order to obtain the thermogram.
- Thermobalance: Sample holder kept inside the furnace is attached with the thermocouple to observe the change in weight and temperature.
- Recorder: Thermogram is obtained by recording the change in weight on Y-axis and temperature on X-axis
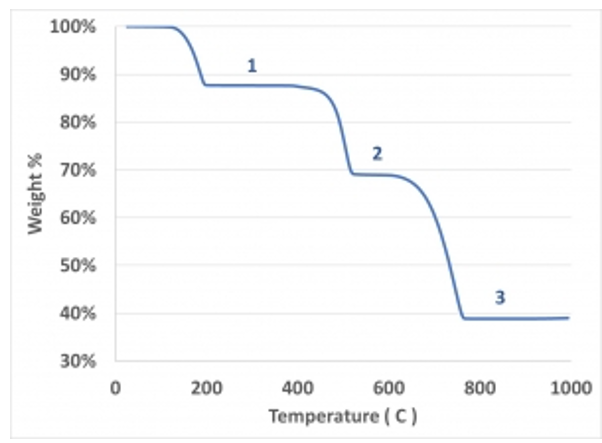
TGA of Calcium oxalate monohydrate (CaC2O4.H2O)
• The successive plateau corresponds to the formation of anhydrous salt, calcium
carbonate and calcium oxide.
(1) CaC2O4.H2O → CaC2O4 + H2O
(2) CaC2O4 → CaCO3 + CO
(3) CaCO3 → CaO + CO2
• The thermogram indicates that the loss of water begins at 100°C and loss of CO at 400°C and CO2 at 680°C.
Now that you have read about Thermogravimetric analysis. Check out our course on Ampersand Academy Read this interesting article about Thin Film Deposition.
