know in detail about the preparation storage and preservation of laboratory samples for food analysis is explained
Preparation of Samples for Food Analysis
The preparation of the samples for food analysis involves reducing the amount and simultaneous reduction in particle size by thorough mixing.
To obtain precise analytical results, the laboratory samples for food analysis must be made as homogeneous as possible so that the results are reproducible within the limits of the analytical method used.
The method of homogenisation will depend on the type of food being analysed. Usually, the samples for food analysis are abstracted from different portions of the food material, mixed and blended, and the representative sample is taken.
A number of very effective electrical, mechanical devices are available to slash the size of food particles and to mix food products thoroughly, mincers, graters, blenders and homogenisers for dry, moist, and wet foods, and several types of powder mills or grinders are all essential pieces of equipment.
Mechanical devices produce heat, so care must be taken not to alter the sample’s composition by losing moisture due to overrunning the equipment.

Dry foods
Pulses and cereals
The sample for food analysis is cleaned free of sand, stone and other extraneous material and powdered. The size of powdery food can be reduced by a process called Quartering.

Flesh foods
Either the whole tissue or a representative protein is minced and then blended with double its weight of water. Portions of the slurry are then removed into weighed containers which are then reweighed to determine the amount of tissue used.

Fluid foods
The top or bottom-drive blenders best emulsify the sample for food analysis.
Oils and fats
They are prepared by gentle warning and mixing. Faty mixed-phase products such as cheese, butter, margarine and chocolate are challenging to be prepared by gentle warming and mixing. Cheese and chocolate are best grated, followed by hand mixing of grated material. Butter and margarine may be re-emulsified by blending by hand in a glass jar after warming to 35ºC to melt the fat.

Fruits and vegetables
The samples for food analysis are taken small in size. However, big ones should be cut, and portions of the edible material should be used. The non-edible portions are rejected, and the edible portions are cut into small pieces. Samples are taken from the mixture of these pieces by Quartering. It is preferable to use stainless steel trays, knives and scissors for preparing the sample.

Prepared samples for food analysis may change composition through evaporation or absorption of moisture or by the action of enzymes or microorganisms. The components which are likely to change should be analysed immediately after preparation.
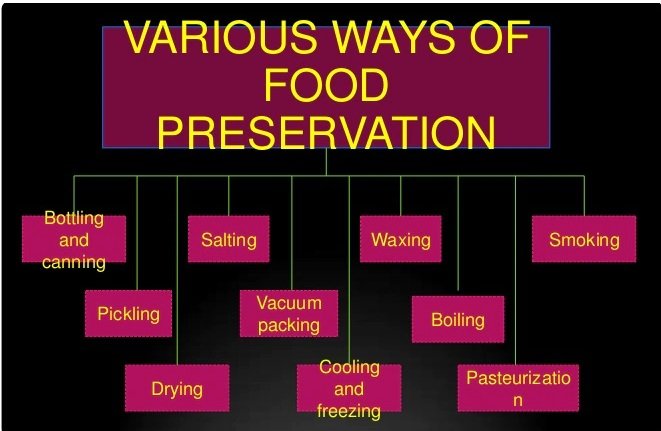
Preservation of samples for food analysis
The prepared samples for food analysis are stored in hermetically sealed inert containers like glass jars or wide-mouthed bottles with screw caps or friction top tin containers.
The dried fruits and fruit products containing 20-30% moisture are stored in glass or plastic containers.
Hydrolytic changes caused by the enzymes may be prevented by dropping the sample into boiling alcohol. The alcoholic extracts are stored below 0ºC to avoid changes in composition.
Products likely to undergo microbial spoilage may be preserved using acetic acid or sodium benzoate as a preservative by freezing or drying.

Freezing of samples for food analysis
Freezing of samples for food analysis in air and moisture-proof containers by rapid freezing and storage at less than -6ºC prevents microbial activity, but not enzyme activity, which continues to occur although at a slower rate temperature down to -40ºC.
Storage of dried products at 0ºC to 10ºC minimises deterioration. While taking samples stored at low temperature for analysis, either the entire sealed container should be warmed to room temperature or a portion transferred quickly to a clean, dry stoppered container to avoid changes in moisture content.

Moisture
Water in plant foods may occur in any of three different forms
- As a dispersing medium for the colloids and as a solvent for crystalloids, i.e. as free water.
- It may be absorbed on the surfaces of colloidal particles in the protoplasm, the cell walls, and cell constituents such as proteins, starches, cellulose which hold water tenaciously.
- As the water of hydration in chemical combination, with various substances like carbohydrates and hydrates of salts.
The “bound water” found in biological materials and the water in colloidal systems may exist as
- Occluded water
- Capillary water
- Osmotic water
- Colloidal water bound by physical forces
- Chemically bound water
For more details about various courses, check out Ampersand Academy.
